|
|
||||||||||||||||||||||||||||
|
Chemical and Process Engineering |
||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||||||||||
|
Fluorocarbon Distillation |
||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||||||||||
|
Project Background |
|
|
|
|
End Client: F2 Chemicals, UK |
|||||||||||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||||||||||
|
F2
Chemicals are an established manufacturer of high specification
perfluorocarbons for a variety of industries including high purity products
for use in semiconductor sector. In
2011, JBL provided process engineering services to ETDE Contracting for a
design study they were undertaking for F2 Chemicals for the production of a
hypergolic halogen based compound. Subsequently JBL was invited by the
contractor, now Bouygues Energies and Services, to undertake a series of
flowsheeting studies for the distillation of Octafluoropropane manufactured
by F2 Chemicals in Preston UK. Octafluoropropane (also known as perfluoropropane, R218
or just 218 is manufactured by F2 Chemicals and marketed as Flutec PP30). It
is used extensively by the semiconductor industry in etching processes and
chemical vapour deposition (CVD) chamber cleaning to remove dielectric film
build up. Octafluoropropane,
C3F8 (PP30), is manufactured by the indirect reaction
of fluorine gas (F2) with Hexafluoropropylene, C3F6
(HFP). The process is carried out in a
reactor followed by distillation. An
improved continuous distillation process was required to replace the current
batch distillation unit. The new distillation plant was required to have an
increased capacity, achieve higher product purity and reduced emissions. The
project also required a new dedicated HFP storage area and product filling
facility. The studies were undertaken in four
phases from 2013 to 2015 and subsequently a facility was designed, built and
finally commissioned in 2017. JBL was
engaged to provide process engineering services throughout the project. The
facility also included Hexafluoropropylene bulk tanker off-loading, reagent
storage, handling and reactor feed. |
Photo courtesy of F2
Chemicals Limited |
|||||||||||||||||||||||||||
|
Flowsheeting
Studies JBL engaged the services of a capable
flowsheet modeller to undertake the flowsheet simulation of the distillation
process and associated vapour recovery unit. JBL worked with F2 Chemicals and
the contractor to provide the modeller with a specification of requirements.
JBL liaised with the modeller throughout each of the study phases. The main
objective was to change from the current batch distillation achieving 99.99%
product purity to continuous distillation (in campaigns) achieving 99.999%
product purity. The new process would also need to reduce emissions of
Octafluoroproane. Phase 1 This
report presents the results on the initial plate-to-plate simulation for
PP30-Distillation and associated vapour recovery unit. The simulation used
the NRTL activity co-efficient physical property package as this is
recommended for chemical systems. A sense check was made using equation of
state methods. The flash calculations for vapour recovery unit were in close
agreement. For distillation, the only property methods that were able to
generate results were activity coefficient methods similar to NRTL. Equations
of state methods were not able to predict vapour-liquid equilibrium. The NRTL
method is preferred due to better prediction of liquid phase mixture
properties. |
||||||||||||||||||||||||||||
|
Phase 2 The
project specifications for this phase of study included two new components
added as part of impurities in the feed stream. One of these components was
identified to have tendency for azeotrope formation with the main product.
The level of this impurity in the distillation feed would render the 99.999%
purity target unachievable. It also identified the risk of impurity
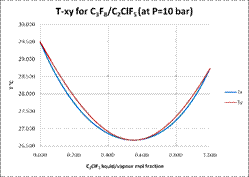
accumulation in the vapour recovery system. Binary data was presented to
illustrate the problem. The report presented a number of options to resolve
this issue. |
|
|||||||||||||||||||||||||||
|
Phase 3 Following a
review with F2 Chemicals raw material with an acceptably low level of contaminant
was sourced for the fluorination reactor. The flowsheeting exercise was
re-run to enable the required product purity to be achieved. Phase 4 This
phase of study was primarily different to the previous phases in terms of the
underlying objectives. The previous phases were mainly aimed at finding
optimal operating conditions and packed bed dimensions to achieve required
purity targets. However, this study was aimed at validating the work carried
out in past studies for the pre-selected packed bed dimensions based on
providing a practical design. The following list represents the main changes
introduced to simulation for this phase: · Increased
diameters from the theoretical requirements for both distillation columns in
order to minimise wall effects. · Increased
pressure in the first distillation column to enable feed to the second column
by differential pressure. · Increased
packing heights to make full use of the available height agreed during
planning permission. Site
Activities · Liaison with
F2 Engineering and Production Personnel · Redefinition
of Control Sequences · Assistance
with FATs and later SATs during water commissioning. · Prestartup
Safety Reviews (PSSR) · Water Commissioning · Degreasing · Chemical
Commissioning · Operator
Training |
||||||||||||||||||||||||||||
|
|
|
|||||||||||||||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||||||||||
|
|
|
|||||||||||||||||||||||||||
|
. |
|
|
|
|
||||||||||||||||||||||||
|
|
|
|
||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||||||||